UDMH + N2O4 AS A FULE USED FOR THE SECOND STAGE: GS2
In the second stage of the GSLV engine,
‘VIKAS’ is used for its functioning. This stage was derived from the PS2 of PSLV and it was the best place where the ‘Vikas engine has proved its reliability. This engine can exert Thurs up to 800km and the Burn time of this engine is 150 sec. it uses 37.5 metric tons of liquid propellants. In which the
UDMH as a fuel and nitrogen tetraoxide (N2O4) is an oxidizer. This UDMH is a stable fuel for long periods. This unique specification makes this rocket engine, but its cost is high so that many cheap missions can’t afford it.
This propellant became the storable liquid propellant of choice in the late 1950s. And the unsymmetrical Dimethylhydrazine (CH3)2NNH2 also became the storable liquid fuel of choice by the 1950s. The development of this UDMH has begun in the Soviet Union in 1949. In the United States, it is used in the majority number of satellites. This was also used in virtually all storable liquid rockets except for some maneuvering engines. The MH was preferred in the fuel due to its slightly higher density and performance.
Some of the specifications of this fuel are as follows.
1 –
Specific Impulse, it has the specific impulse of 333s.
2 – Specific impulse sea level, at the sea level this is 285s.
3 – Location, the location of this fuel is 1720.
Including the above details, I would like to add some more specifications related to this fuel and the engine. These specifications are like the
optimum Oxidizer which is present in the fuel is 2.64 and the temperature
of combustion of the fuel is 3,415 degrees k, including the specific heat is 1.25, and the Density is 1.18 g/c, the characteristic velocity is 1720m/s (5,640 feet/sec). Oxidizer density 1.450 g/cc and the Oxidizer freezing point -11degree c, the fuel density is 0.793 g/cc, the fuel boiling point is 63 degrees Celsius. This is described as a specific fuel in the United States in the form of MIL-PRF-25604.
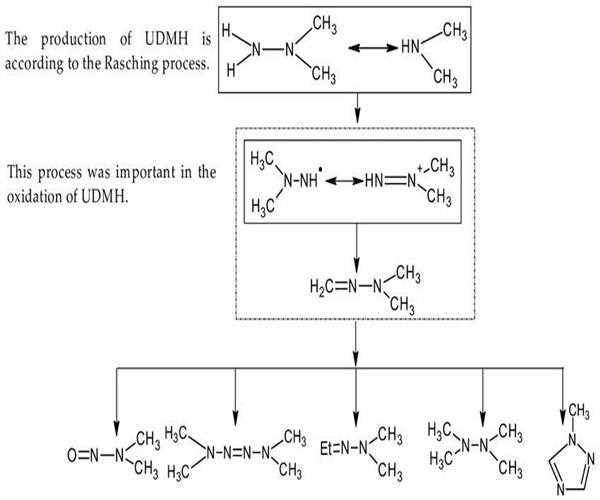
PRODUCTION OF UDHM
This fuel is produced in a large quantity with the help of the two ways. These methods are based on the same concept of reaction of monochloramine with dimethylamine.
1- (CH3)2NH + NH2CL------- (CH3)2NNH2.HCL
2- CH3C(O)NHNH2+2CH2o +2H2-----CHC(O)NHN(CH)+2NH
CHC3C(O)NHN(CH3)2+H2O----CH3COOH+H2NN(CH3)2 .
