The size of an atom is one of the most important factors that determines the strength of a chemical bond. In general, smaller atoms have stronger bonds than larger atoms. This is because the nucleus of a smaller atom is more tightly held by the electrons, which makes it more difficult to break the bond.
There are a few reasons why the size of an atom affects the strength of a chemical bond. First, the smaller the atom, the closer the nucleus is to the electrons. This means that the attractive force between the nucleus and the electrons is stronger, which makes it more difficult to break the bond.
Second, the smaller the atom, the less shielding there is from the nucleus. This means that the electrons are more exposed to the positive charge of the nucleus, which makes them more likely to be attracted to the nucleus and less likely to be shared with another atom.
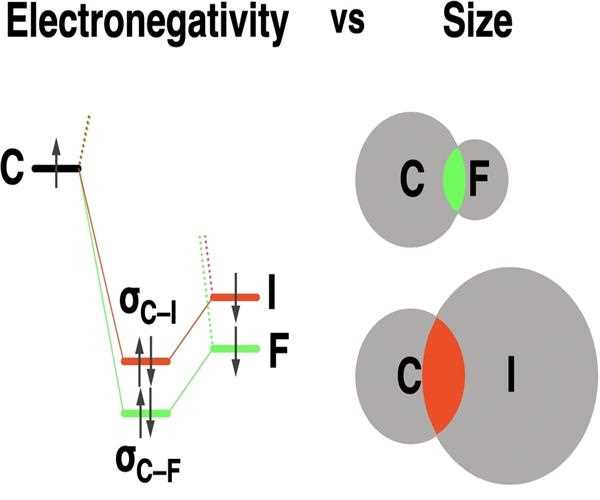
Third, the smaller the atom, the less polarizable it is. This means that it is less likely to have its electron cloud distorted by the presence of another atom. This makes it more difficult for the electrons to be shared between the two atoms, which weakens the bond.
The relationship between the size of an atom and bond dissociation can be seen in the following examples:
- The bond between hydrogen and fluorine is one of the strongest bonds in nature. This is because both hydrogen and fluorine are small atoms, which means that they have strong attractive forces between their nuclei and their electrons.
- The bond between carbon and hydrogen is also relatively strong. This is because carbon is a smaller atom than hydrogen, which means that the attractive force between the nucleus and the electrons is stronger.
- The bond between carbon and chlorine is weaker than the bond between carbon and hydrogen. This is because chlorine is a larger atom than hydrogen, which means that the attractive force between the nucleus and the electrons is weaker.
The relationship between the size of an atom and bond dissociation is an important concept in chemistry. It can be used to explain why some chemical bonds are stronger than others, and it can also be used to predict the properties of new compounds.