Hydrogen
In the periodic table, this is the first member and has the lowest atomic number which is 1,
and the symbol representation is ‘H’. This is the lightest element in the periodic table and the standard situation, this is in the form of a
Diatomic molecule which is known as the
Hydrogen gas having the formula of H2. This gas is the most abundant in the universe and it contains about 75% of all the normal matter.
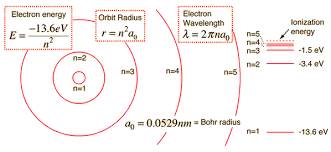
The energy in the lowest state of the atom is -13.6 eV. The energy level of Hydrogen can be easily calculated with the help of the
Bhor model
of the atom. This model explains that the electrons and protons of the atom are bounded by the
electromotive force while in the solar system, all the planets revolve around the Sun due to the reason of the presence of
Gravity.
The most accurate description of the energy of the hydrogen can be seen in the
Schrodinger Equation with the help of quantum mechanics. The electrons of the ground state of the Hydrogen have no momentum.
Orbitals energy in the
Hydrogen atom
As we saw in the electronic configuration section, we see that there are many different energy sections. The lowest energy state has of an atom has its electrons filling orbitals which can have
two electrons in the ground state to achieve the stable state.
When the energy is added to the ground state orbital an electron can go to the one higher stable state of energy orbital. There is a fact which is very necessary for it. and that
the same energy should be added as the energy occupied by the higher state and the lower state and the
difference between both is the requirement in the energy.
Read more